 PNCR
PNCR SCR
SCR SNCR
SNCR Semi-dry system
Semi-dry system Dry system
Dry system Photovoltaic
Photovoltaic Case
Case
Semi-dry system
Position:Home > Semi-dry system >
Semi-dry system
Rotary spray flue gas purification process principle:
The flue gas treatment process is that high-speed centrifugal atomized absorbent is fully contacted and reacted with the acid gas in the flue gas in the semi-dry reaction tower to achieve removal of acid gas and other harmful substances. This allows the incinerator tail gas to be purified in a semi-dry reaction tower. The spray deacidification process is divided into five steps: (1) absorbent preparation; (2) atomization of absorbent slurry; (3) mist droplet contact with flue gas; (4) evaporation-acid absorption; (5) waste residue exclude. Its chemical and physical processes are described below.
Chemical process: When the hydrated lime slurry is atomized in the semi-dry reaction tower through the atomizing nozzle and is in full contact with the flue gas, the flue gas is cooled and humidified, Ca(OH)2 particles in the slurry react with HCL, SO2, etc. By-products are generated, and the reaction products are dried by the heat of the flue gas. The reaction is divided into gas phase, liquid phase, and solid phase. The following reaction formulas illustrate the temperature range at 140-160°C. The nature of gas deacidification (the formula given is a cumulative formula that does not reflect the actual reaction process at a single step).
The flue gas treatment process is that high-speed centrifugal atomized absorbent is fully contacted and reacted with the acid gas in the flue gas in the semi-dry reaction tower to achieve removal of acid gas and other harmful substances. This allows the incinerator tail gas to be purified in a semi-dry reaction tower. The spray deacidification process is divided into five steps: (1) absorbent preparation; (2) atomization of absorbent slurry; (3) mist droplet contact with flue gas; (4) evaporation-acid absorption; (5) waste residue exclude. Its chemical and physical processes are described below.
Chemical process: When the hydrated lime slurry is atomized in the semi-dry reaction tower through the atomizing nozzle and is in full contact with the flue gas, the flue gas is cooled and humidified, Ca(OH)2 particles in the slurry react with HCL, SO2, etc. By-products are generated, and the reaction products are dried by the heat of the flue gas. The reaction is divided into gas phase, liquid phase, and solid phase. The following reaction formulas illustrate the temperature range at 140-160°C. The nature of gas deacidification (the formula given is a cumulative formula that does not reflect the actual reaction process at a single step).
Ca(OH)2 + SO2 = CaSO3*½H2O + ½H2O
Ca(OH)2 + SO3 = CaSO4*½H2O + ½H2O
Ca(OH)2 + H2O + SO2 + ½O2 = CaSO4*2H2O
CaSO3*½H2O + ½O2 = CaSO4*½H2O
Ca(OH)2 + CO2 = CaCO3 + H2O
Ca(OH)2 + 2HCl = CaCl2 + 2H2O
Ca(OH)2 + 2HF = CaF2 + 2H2O
Ca(OH)2 + SO3 = CaSO4*½H2O + ½H2O
Ca(OH)2 + H2O + SO2 + ½O2 = CaSO4*2H2O
CaSO3*½H2O + ½O2 = CaSO4*½H2O
Ca(OH)2 + CO2 = CaCO3 + H2O
Ca(OH)2 + 2HCl = CaCl2 + 2H2O
Ca(OH)2 + 2HF = CaF2 + 2H2O
In the case of HCl in the flue gas, the optimum operating temperature is approximately 15-25°C above the flue gas saturation temperature.

Dust collector and reaction tower
|

Mortar preparation
|
|
Rotary spray method operation screen
|
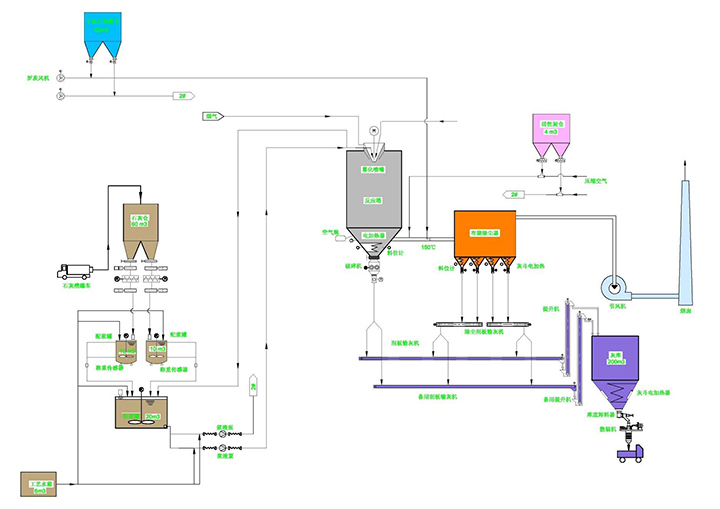
Rotary spray process
|
